The Covid-19 Pandemic is one of the greatest challenges modern medicine has ever faced. Hospitals and research labs all over the world are testing many different therapies on coronavirus-positive patients in an effort to find a potential COVID-19 treatment. There are thousands of clinical trials investigating treatments and preventative measures for COVID-19.
This newsletter will review the prominent drugs, the Israeli achievements, and where Gsap helps promote the fight against Coronavirus.
Topics in this newsletter:
- Treatment of COVID-19
- Promising Israeli drugs to treat Corona virus
- Effects of Pfizer (Tozinameran/Comirnaty) in the Israeli population
Current approaches to COVID-19 therapies generally fall into two categories: antivirals which prevent the virus from multiplying and immune modulators which help the immune system to fight the virus or stop it from overreacting dangerously.
During a public health emergency, such as COVID-19, the FDA can issue an emergency use authorization (EUA) to help make new medications and medical products more available to patients. Having a EUA does not mean that the FDA has approved the medication or product. Rather, the intent of a EUA is to make it easier for patients to receive a new potential treatment when no other options are available.
Treatment of COVID-19
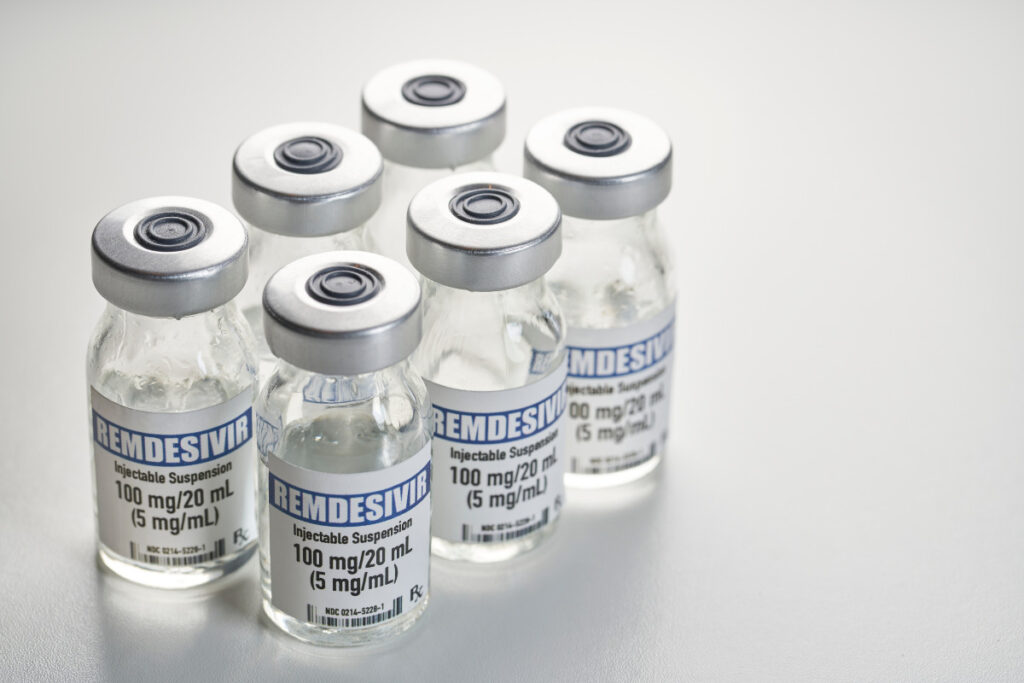
Remdesivir – First drug to gain approval from the
Remdesivir is the first drug to gain approval from the FDA for the treatment of Covid-19 made by Gilead Sciences under the brand Veklury, it works by interfering with the creation of new viruses, inserting itself into new viral genes. Remdesivir was originally tested as an antiviral against Ebola and Hepatitis C, only to deliver lackluster results. But once the Covid-19 pandemic emerged, researchers found that it could stop the coronavirus from multiplying in cells. A large clinical trial was then launched, which found that the drug reduced the recovery time of people hospitalized with Covid-19 from 15 to 11 days. The FDA responded to this data last May by issuing an emergency authorization for Remdesivir’s use in critically ill patients who need supplemental oxygen. In August, they expanded that approval after another study found that patients with less severe forms of Covid-19 seemed to benefit modestly from a five-day treatment course of Remdesivir. The revised approval allows the use of the drug on all patients hospitalized with Covid-19, regardless of how severe their disease is.
Yet many experts remained skeptical of remdesivir’s benefits. They pointed out, for example, that there’s no statistically significant evidence that remdesivir actually prevents deaths from Covid-19. In Nov 2020, the World Health Organization recommended against using remdesivir. Based on a review of all the published trials so far, they concluded that evidence of its benefits is lacking.
FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19
Monoclonal antibodies are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses. Casirivimab and imdevimab are monoclonal antibodies that are specifically directed against the spike protein of SARS-CoV-2, designed to block the virus’ attachment and entry into human cells.
In November 2020, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for basiliximab and imdevimab to be administered together for the treatment of mild to moderate COVID-19 in adults and pediatric patients (12 years of age or older weighing at least 40 kilograms) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19. This includes those who are 65 years of age or older or who have certain chronic medical conditions.
In a clinical trial of patients with COVID-19, casirivimab and imdevimab administered together were shown to reduce COVID-19-related hospitalization or emergency room visits in patients at high risk for disease progression within 28 days after treatment when compared to placebo. The safety and effectiveness of this investigational therapy for use in the treatment of COVID-19 continue to be evaluated.
A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia demonstrate no significant difference between the convalescent plasma group and the placebo group
Convalescent plasma is frequently administered to patients with Covid-19 and has been reported, largely on the basis of observational data, to improve clinical outcomes. Minimal data are available from adequately powered randomized, controlled trials.
The New England journal of medicine published in February 2021 a Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. Adult patients with severe Covid-19 pneumonia in a 2:1 ratio receive convalescent plasma or placebo (A total of 228 patients were assigned to receive convalescent plasma and 105 to receive placebo). The primary outcome was the patient’s clinical status 30 days after the intervention, as measured on a six-point ordinal scale ranging from total recovery to death. On day 30 day, no significant difference was noted between the convalescent plasma group and the placebo group in the distribution of clinical outcomes according to the ordinal scale.
Corticosteroid use in COVID-19 patients
Corticosteroids (often called steroids) are used to tamp down inflammation and for conditions such as allergies and asthma. The Covid-19 pandemic brought a new interest in these drugs, and a raft of new clinical trials was launched. In June 2020, the steroid dexamethasone was the first shown to reduce Covid-19 deaths. A study of more than 6,000 people found that dexamethasone reduced deaths by one-third in patients on ventilators, and by one-fifth in patients on oxygen. It may be less likely to help and may even harm patients who are at an earlier stage of Covid-19 infections, however. In its Covid-19 treatment guidelines, the National Institutes of Health recommends only using dexamethasone in patients with Covid-19 who are on a ventilator or are receiving supplemental oxygen.
In September 2020, researchers reviewed the results of trials on dexamethasone, along with two other steroids, hydrocortisone, and methylprednisolone. Overall, they concluded, steroids were linked with a one-third reduction in deaths among Covid-19 patients.
Promising Israeli drugs to treat Coronavirus 🇮🇱
EXO-CD24
The EXO-CD24 substance, developed at the Ichilov Medical Centre in Tel Aviv, successfully completed its first phase of clinical trials.
CD24 is a small heavily glycosylated GPI-anchored protein. CD24 is a key player in the vast majority of human cancers and also plays an important role in controlling the homeostatic proliferation of T cells. Hence, CD24 can negatively regulate inflammation.
The treatment is a biologic therapeutic agent based on exosomes carrying CD24. The rationale for this treatment is that exosomes overexpressing CD24, isolated and purified from T-REx™-293 cells engineered to express CD24 at high levels, can suppress the cytokine storm and are delivered directly to the target organ (the lungs) using exosomes as a highly body-compatible delivery vehicle. This enables a strong reduction of the required dose and reduces the risk for adverse events.
The treatment was administered to 30 patients with moderate-to-severe symptoms of Covid-19. Twenty-nine of them recovered in up to five days. No placebo was used in the first stage of the trial, and the next phase of the clinical trials will continue to examine the effects and efficacy of the treatment.
During a recent visit to Israel, Greek Prime Minister Kyriakos Mitsotakis offered to have a hospital in Greece take part in clinical trials.

Allocetra
Israeli immunotherapy company Enlivex Therapeutics reported positive interim results of Phase II clinical trial of its Allocetra product in severe and critical Covid-19 patients. The interim clinical results relate to eight Covid-19 patients who were treated with Allocetra, six of whom were in severe condition and two of whom were in critical condition. Seven completely recovered and were discharged from the hospital, after an average of 4.7 days following Allocetra administration. The eighth treated patient in the Phase II study has experienced a clinical improvement following treatment with Allocetra and is currently classified as a moderate/severe condition. The company says that the Allocetra treatment has been well tolerated with no treatment-related serious adverse events. On December 3, 2020, the Company reported positive interim results of Phase II investigator-initiated clinical trial of Allocetra in COVID-19 patients in severe/critical condition.
The interim clinical results relate to eight COVID-19 patients who were treated with AllocetraTM in Phase II clinical trial, six of whom were in severe condition and two of whom were in critical condition. Key results and conclusions from both the ongoing Phase II clinical trial, as well as a previously-reported investigator-initiated Phase Ib study include:
- Seven out of seven (100%) patients treated through November 26, 2020 had complete recovery from their respective severe/critical condition and were discharged from the hospital, after an average of 4.7 days following AllocetraTM administration.
- Taken together with previously-treated patients in the concluded Phase Ib study, twelve out of twelve patients (100%) through November 26, 2020 had complete recovery from their respective severe/critical condition and were discharged from the hospital, after an average of 5.5 days following AllocetraTM administration.
- The eighth treated patient in the Phase II study (and 13th treated patient overall), who enrolled in the Phase II study in critical condition on November 27, 2020, has experienced a clinical improvement following treatment with AllocetraTM and was classified as moderate/severe condition on December 3, 2020. Clinical outcome will be included in the next interim results update.
- AllocetraTM treatment has been well tolerated with no treatment-related serious adverse events.
Corona virus vaccines news
Vaccines typically require years of research and testing before reaching the clinic, but in 2020, scientists embarked on a race to produce safe and effective coronavirus vaccines in record time. Researchers are currently testing 71 vaccines in clinical trials on humans, and 20 have reached the final stages of testing. At least 78 preclinical vaccines are under active investigation in animals.
Effects of Pfizer (Tozinameran/Comirnaty) in the Israeli population
In November 2020 Pfizer and the German company, BioNTech made history by announcing that their coronavirus vaccine had an efficacy rate of over 90 percent, far surpassing expectations. It was the first time anyone had found such evidence. Just over a month later, in December 2020, the Food and Drug Administration granted it the first emergency use authorization ever given by the United States to a coronavirus vaccine.
In January 2020, BioNTech researchers started molding a genetic molecule called messenger RNA (mRNA) which create the genetic instructions for building a coronavirus protein, known as a spike. When injected into cells, the vaccine causes them to make spike proteins, which then get released into the body and provoke a response from the immune system. In May a clinical trial was started.
In Phase 1 trials, the researchers found that Comirnaty caused volunteers to produce antibodies against SARS-CoV-2, as well as immune cells called T cells that respond to the virus. In July 2020, the companies announced the launch of a Phase 2/3 trial with 30,000 volunteers. In September, Pfizer and BioNTech announced that they would seek to expand the trial to 44,000 participants, and in November 2020, Pfizer and BioNTech released a preliminary analysis of the first 94 cases. Comirnaty has an efficacy rate of 95 percent. While Comirnaty caused no serious side effects, it frequently caused short-lived fatigue, fever, and muscle aches. These impressive results led rapidly to authorizations across the world.
Israel is currently leading the world in vaccination. Israel vaccinated 53.7% of its 9 million inhabitants, with at least the first dose of the vaccine (Until the first of March(. Pfizer and Israeli health officials released new data that shows that the Comirnaty vaccine is greatly reducing transmission, which is one of the most asked questions in the world right now. The Israeli Health Ministry found that the full two doses of Pfizer reduce infection by 89.4% in asymptomatic cases, where there are no visible or tangible symptoms. In cases that bring symptoms, Pfizer seems to work to provide a startling 93.7% level of protection. The vaccine was also 92% effective at protecting people from severe illness after two shots, with a strong 62% protection level after a single dose. Three weeks after the first dose, people reported a 72% level of protection. Scientists expect this percentage to increase over time, as immunity builds in the body.
F.D.A. Panel Gives Green Light to Johnson & Johnson’s Vaccine
Johnson & Johnson’s Covid-19 vaccine was endorsed at the end of February 2021 by a panel of experts advising the Food and Drug Administration, clearing the last hurdle before a formal authorization.
Johnson & Johnson’s formulation worked well in clinical trials, particularly against severe disease and hospitalizations, even though it did not match the sky-high efficacy rates of the first two vaccines made by Pfizer-BioNTech and Moderna.
Johnson & Johnson launched a Phase 3 trial in September, which they paused on Oct. 12 to investigate an adverse reaction in a volunteer. The trial resumed eleven days later. Although Johnson & Johnson initially set out to recruit 60,000 volunteers, it capped the trial at 45,000 in December as cases rose.
Institute for Biological Research start second trial phase for COVID-19 vaccine
The Israel Institute for Biological Research announced the start of phase two with a vaccine for the COVID-19 virus (IIBR-100/ BriLife). The study was started with a dose-escalation phase (phase I) during which subjects (18-55 years old) were randomly allocated to receive a single administration of BriLife at low, mid, or high dose or saline or two administrations of IIBR-100 at a low dose, or saline, 28 days apart.
Based on results obtained during phase I, and cumulative phase I data review, the expansion phase (phase II) was started, during which larger cohorts, as well as elderly age subjects, were randomly allocated to receive prime-boost administration of BriLife 28 days apart. The subjects will be followed for a period of up to 12 months post-last vaccine administration to assess the safety and efficacy of the vaccine.
Gsap has the honor to accompany the Israel Institute for Biological Research through the various stages of development and clinical trials.
This Newsletter Prepared by:

Sara Blumenstein Pharma & Biotechnology Regulatory Section Manager